
testing for urinary tract infections (utis)
Utilizer-ID test is easy to use for health care professionals in a near patient setting. The product is based on the gold standard culture using chromogenic agar.
Utilizer-ID test is identifying the five most common bacterial pathogens in urine; E.coli, Klebsiella Pneumoniae, Enterococcus Faecialis, Proteus Mirabillis and Staphylococcus Saprophyticus within the concentration interval 1,000-100,000 CFU/mL to support diagnosis or screening of urinary tract infection. The test is based on phenotypic testing allowing all the strains of the five bacteria to be identified using an innovative miniaturization technology combined with chromogenic agar and digital readout.
The hands-on time is around two minutes followed by an incubation of 5-8 hours. The test result is then read by a smartphone or mobile device within one minute and the results uploaded to a connected Laboratory Information System (LIS) or Hospital Information System (HIS) systems when required.
The product is currently in clinical validation in Sweden, whereafter it will be CE marked for European conformity according to In Vitro Diagnostic Regulation (IVDR).

how utilizer’s id test works
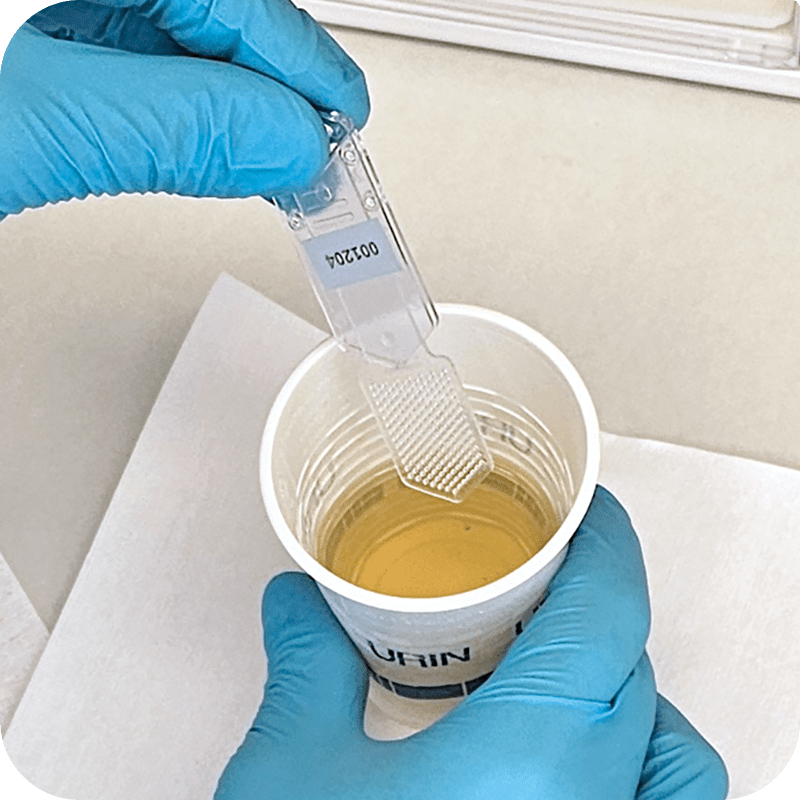
step 1
Dip the Utilizer-ID dipstick in patient’s urine sample

step 2
Incubate dipsticks at 36°C±2°C

step 3
Photograph dipstick and upload to smartphone

step 4
Automated analysis and real time connectivity
Utilizer test provides clinically relevant quantification range for urinary tract infections. Sensitivity range 103 – 105 CFU / mL.

antibiotic susceptibility testing (last)
Our second product in development is an innovative AST test for urinary tract infections, designed to be used alongside our Utilizer-ID product in primary care settings.
This test aims to significantly reduce the time to results, enabling faster, informed treatment decisions. Patients no longer need to wait 2–5 days for traditional laboratory results—treatment can begin much sooner.
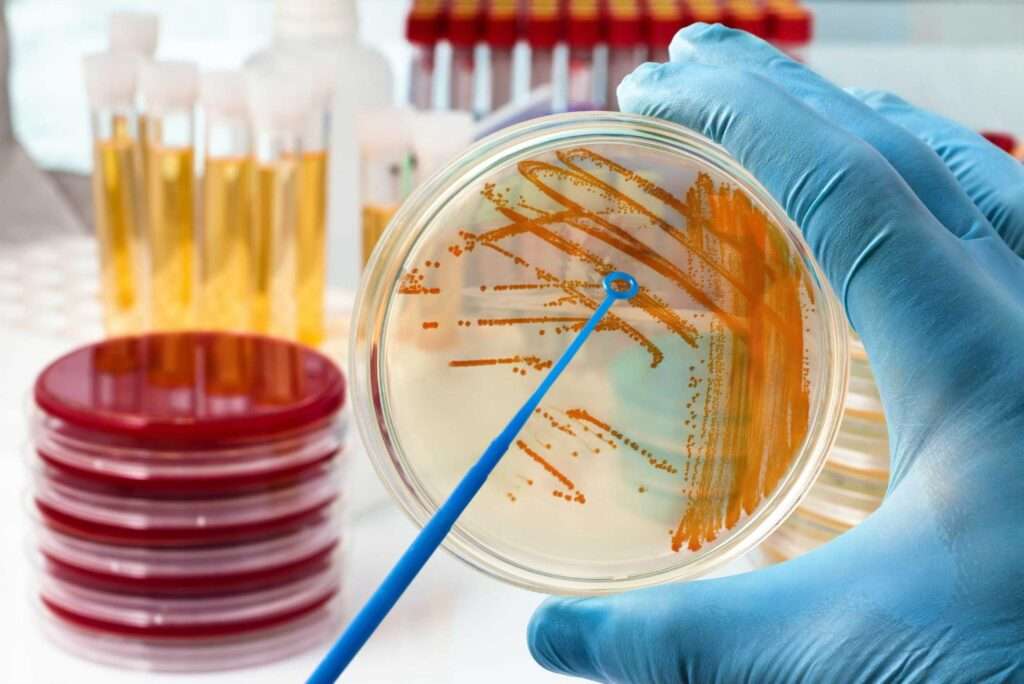

other types of infection
Our technology enables many tests to be developed for a multiple different areas, such as veterinary testing, home testing and food hygiene control.

home testing kit
Bacteria panel with urine sampling device and mini-incubator

food hygiene control
Detection and identification of pathogens in food, dairy and industrial hygiene products

home testing kit
Detection, identification and quantification of pathogens in urine of canines and felines
poc bacteria testing
Detection, identification and quantification of pathogens in respiratory system, etc.
std panel
At home detection of STDs including chlamydia, gonorrhoea, and syphilis

